Empirical Formula of Ascorbic Acid
An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. A calibration curve is one approach to the problem of instrument calibration.
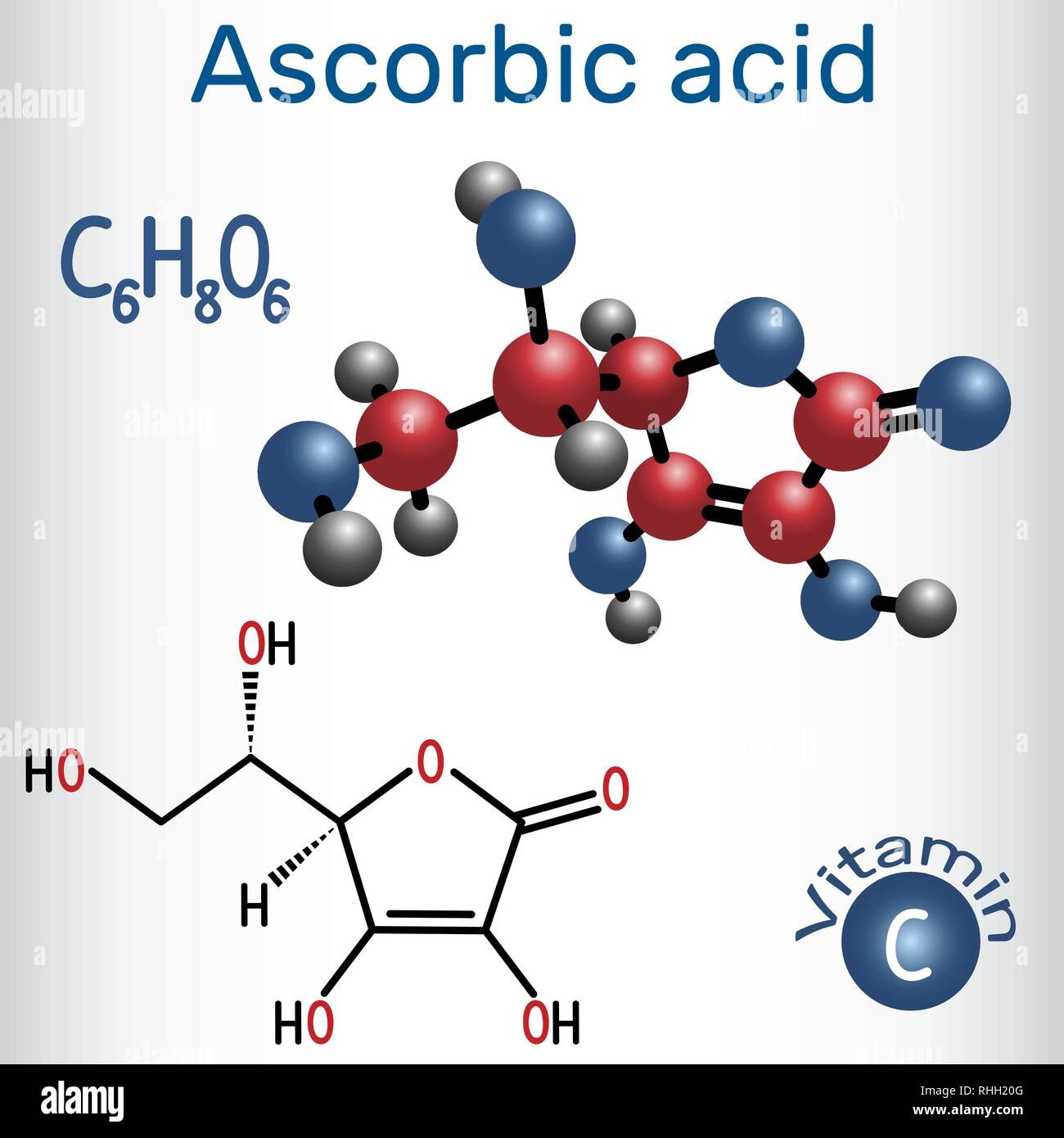
Ascorbic Acid Vitamin C Structural Chemical Formula And Molecule Model Vector Illustration Stock Vector Image Art Alamy
In this context the empirical rule of Reinhoudt for unidirectional energy transfer is significant which states that the energy gap ΔE ST between the excited triplet state T 1 and excited singlet state S 1 of a linker should be higher than 5000 cm 1 and the energy gap ΔE TD between the T 1 of a linker and the excited state of a Ln 3 ion should generally be greater than 3500 cm.

. The empirical formula is C6H806 and the molecular weight is 17613. The empirical formula of Simvastatin is C 25 H 38 O 5 and its molecular weight is 41857. The chemical name of ascorbic acid vitamin c is L-ascorbic acid vitamin c.
A pH of 7 is regarded as neutral. The ingredient may be used in infant formula in accordance with section 412g of the Federal Food Drug and Cosmetic Act the act or with regulations promulgated under section 412a2 of the act. Besides it was determined that Al 3 and Cu 2 metal ions activated the enzyme and the PBPPO was strongly inhibited by ascorbic acid with a K i constant of 167 035 µM.
Nitric Oxide NO Activity. The empirical formula of cyanocobalamin is C 63 H 90 N 14 O 14 PCO. Ascorbic acid butylated hydroxyanisole citric acid monohydrate hydroxypropyl cellulose hypromellose iron oxide yellow isopropyl alcohol lactose monohydrate magnesium.
Inhibitor-enzyme interactions were examined by molecular docking studies and it was revealed that ascorbic acid had the lowest docking score of 654 kcalmol. Compound 4a from the 134-oxadiazole series. The higher the associated electronegativity the.
In analytical chemistry a calibration curve also known as a standard curve is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. Number of moles of a substance from its given mass is Number of moles Given mass. Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen.
Its structural formula is. Vitamin C is known chemically by the name ascorbic acid. Here we will study the pH value formula and how pH value is calculated in detail.
Today vitamin C identical to that occurring in nature. The chemical formula for methyl tert-butyl ether the clean-fuel gasoline additive MTBE is question_answer Q. Enter the email address you signed up with and well email you a reset link.
Other standard approaches may mix the. The corrin ring is almost similar to the tetrapyrrole ring structure found in other porphyrin compounds eg. The screening of the present compound series for NO radical scavenging activity identified excellent NO scavengers.
The synthesis of ascorbic acid was achieved by Reichstein in 1933 followed by industrial production of ascorbic acid two years later by Roche. The empirical formula and the molecular formula of the ascorbic acid should be identified when the combustion of 28 1g ascorbic acid produces 11 5g of H 2 O and 42 1g of CO 2. Electronegativity symbolized as χ is the tendency for an atom of a given chemical element to attract shared electrons or electron density when forming a chemical bond.
Citric acid may be produced by recovery from sources such as lemon or pineapple juice. For the production of ascorbic acid vitamin C the reader must. The compounds FRAP activity was four-fold superior to standard compound ascorbic acid IC 50 10262 µM and comparable to gallic acid IC 50 1785 µM.
Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen. Combustion of hydrocarbon produces CO 2 and H 2 O. Acidity is defined as a pH of less than 7.
The structure of vitamin B 12 consists of a corrin ring with a central cobalt atom. The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution. A pH of more than 7 is classified as basic.
D Prior sanctions for this ingredient different from the uses established in this section do. Heme with Fe and chlorophyll with Mg. 40 mg or 80 mg of Simvastatin and the following inactive ingredients.
Some rockets in the 1960s used hydrazine N2H4 as a fuel and nitric acid HNO3 asan oxidizer.
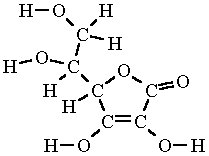
Ascorbic Acid Formula Structure

General Chemistry I Solving For An Empirical Formula Youtube


No comments for "Empirical Formula of Ascorbic Acid"
Post a Comment